IT Solutions for Retail and the Consumer Goods Industry
Successful Retail(ing) Through Digital Transformation and Innovation
We Support Your Value Creation Process in Modern Retail
Digitalization is an important and forward-looking task for retail companies and consumer goods manufacturers. Rising logistics costs, volatile supply chains, a shortage of skilled workers, and changing customer needs require new, digital answers. At the same time, modern IT solutions for retailers offer enormous opportunities to optimize processes, reduce costs, and strengthen customer loyalty in the long term.
Whether ERP system for retail, warehouse management systems or CRM software – we support you with scalable, industry-specific technologies along your entire value creation process. Our expertise ranges from e-commerce platform integration and the development of a successful omnichannel strategy to the optimization of online shops and personalization through AI-supported tools in e-commerce.

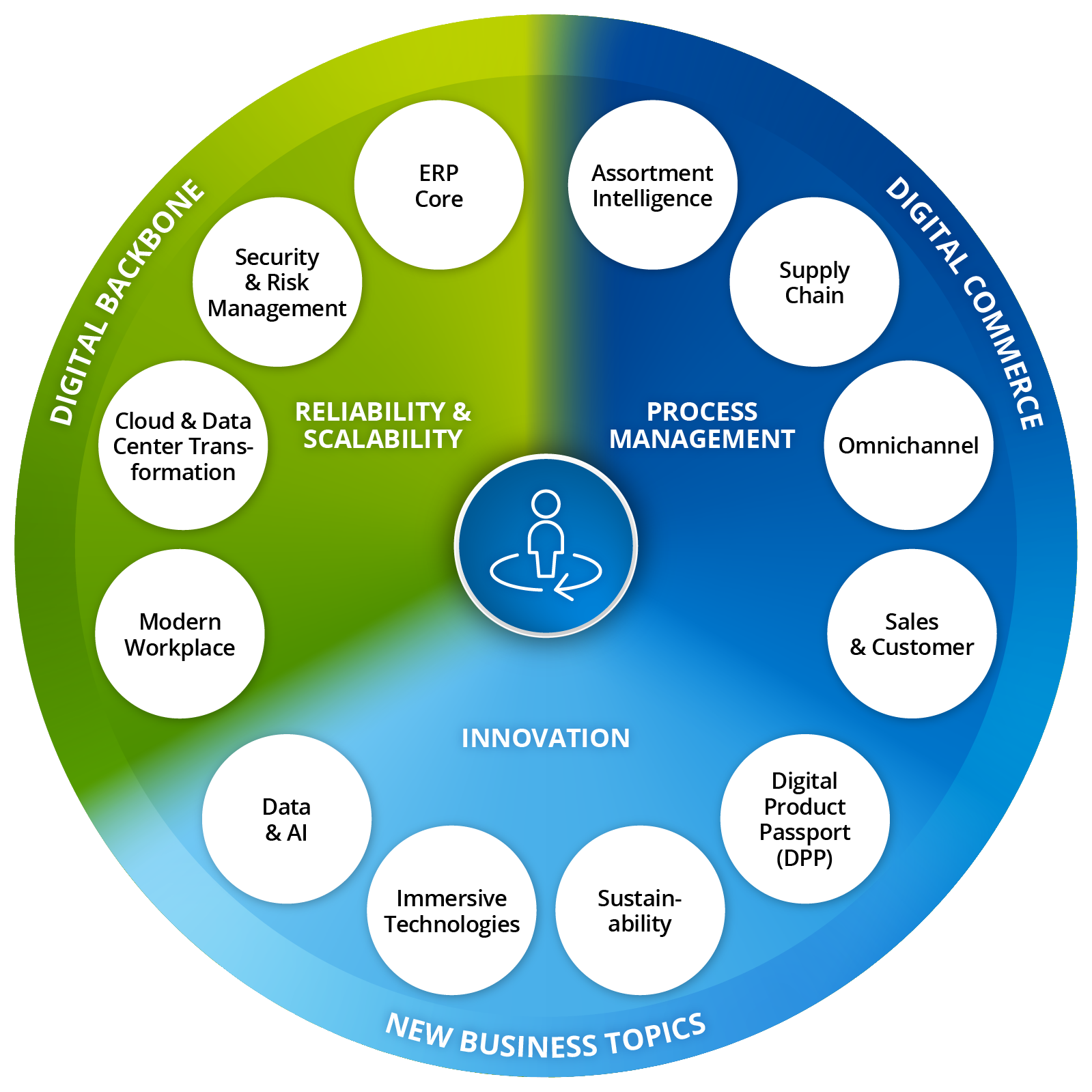
As your partner for Digital Transformation in retail, we provide you with holistic support – from strategic consulting to implementation and operation. With a strong digital backbone and innovative solutions, we create the basis for future-proof business models. Invest now in intelligent IT systems to automate your processes, strengthen your brand, and delight your customers with a seamless, personalized shopping experience – online and offline.

"Arvato Systems is much more than a service provider - it is a trusted partner and advisor. We have a deep connection with the company, and it has been essential in helping us demonstrate how AWS can support our evolving business model."
Certifications
Rely on us: information security, quality management, IT service management. For our customers, we regularly have our performance measured and extensively certified. You benefit in the long term from our adherence to the highest quality standards and our technological expertise in the form of a trusting and highly professional collaboration.
Worth Knowing about the Digital Transformation in Retail and the Consumer Goods Industry
-
What advantages does cloud transformation offer retail companies?
A cloud transformation enables retail companies to modernize their IT infrastructure, automate processes and react more flexibly to market changes. Central data storage and scalable cloud solutions enable companies to work more efficiently, reduce costs and create personalized customer experiences.
-
How do I choose the right ERP software for my retail company?
The selection of an ERP system depends on your business processes, your company size and your integration requirements. Pay attention to industry-specific functions, scalability and interfaces to existing systems such as POS or warehouse management. A well-founded needs analysis and an experienced implementation partner are crucial for success.
-
How do I implement a successful omnichannel strategy in retail?
A successful omnichannel strategy combines online and offline channels to create a seamless customer experience. This requires a central database, integrated systems such as CRM, POS and e-commerce platforms and a clear customer journey. Find out more about the omnichannel solutions from our in-house Unified Commerce Suite aroma® here.
-
How can I reduce high abandonment rates in e-commerce?
Optimize loading times, simplify the checkout process, and rely on personalized product recommendations through AI. Transparent delivery information and a mobile-friendly design increase conversion rates and sustainably reduce abandonment rates.
-
How do I optimize logistics processes in retail sustainably?
By using logistics software such as Warehouse Management Systems (WMS) and Transport Management Systems (TMS) can make supply chains more efficient. Real-time data and AI-supported forecasts help to avoid bottlenecks, shorten delivery times, and minimize delivery delays through IT solutions.
-
What role does artificial intelligence play in the digital transformation of retail?
AI supports retail companies in automating processes, optimizing inventories, and personalizing customer approaches. It recognizes patterns in purchasing behaviour, improves product recommendations, and increases conversion rates. Ready for AI in retail? Get to know our AI companion program here.
-
How do CRM systems and personalization improve the customer experience in retail?
Modern CRM systems enable a targeted customer approach across all channels. In combination with personalized recommendations, this creates a consistent, individual shopping experience that strengthens customer loyalty and increases satisfaction. Here you will find our solutions for a perfect customer experience - from UX design to CRM system.
-
How do immersive technologies improve the customer experience in retail?
Immersive technologies such as augmented reality (AR), virtual reality (VR) and the metaverse are revolutionizing the shopping experience in retail. They enable customers to experience products virtually, interact in digital showrooms or receive personalized advice with the help of avatars - regardless of time and place. For retail companies, immersive technologies offer new opportunities for differentiation. They increase customer loyalty, promote brand awareness and create innovative touchpoints along the customer journey. In this blog article share experiences and a look into the future of online retail.
Your Contact for Retail & Consumer Goods